riboflavin tablet price
Beadlets Lutein Zeaxanthin Lycopene Microencapsulation — field notes from the carotenoid aisle If you’ve followed carotenoids the past few years, you’ve seen the shift: formulators want clean-label, vegetarian beadlets that disperse in cold water yet stand up to tableting pressure. To be honest, it was overdue. The uptick in ophthalmic and healthy-aging SKUs made it inevitable. This is where Beadlets Lutein Zeaxanthin Lycopene Microencapsulation gets interesting—free-flowing, gelatin-free spheres that behave nicely in the real world, not just in PowerPoint. What’s trending and why it matters Three trends collide here: cold-water dispersibility for better bioavailability, microencapsulation for oxidative stability, and vegetarian systems that ditch gelatin. In fact, many customers say they switched because “powders stopped clumping and tabs stopped chipping.” It sounds small. It isn’t. Technical snapshot Beadlets Lutein Zeaxanthin Lycopene Microencapsulation are spherical, free-flowing particles designed for capsules and tablets. The carrier system typically uses plant-based matrices with antioxidants, enabling good compressibility and sustained color integrity. Parameter Typical spec (≈) Method / Standard Lutein content 10–20% w/w HPLC, USP monograph/EP Zeaxanthin content 2–5% w/w HPLC, AOAC 2016.13 Lycopene content 5–10% w/w HPLC, USP/FCC Particle size 20–40 mesh, free-flowing USP Sieve Dispersibility T90 ≤ 30 s in 200 mL water (20°C) In-house, per AOAC guidance Heavy metals Pb ≤ 1 ppm; As ≤ 1 ppm; Cd ≤ 0.5 ppm; Hg ≤ 0.1 ppm ICP-MS, USP Shelf life 24 months, ≤25°C, ≤60% RH ICH Q1A(R2) Process flow (how it’s made) Materials: carotenoid oleoresins, plant-based carrier matrix, antioxidants, emulsifiers, purified water. Methods: pre-emulsification → high-shear homogenization → microencapsulation via spray-drying and fluid-bed layering → antioxidant fortification → classification → metal detection. Stability verified by accelerated testing (40°C/75% RH, 6 months; carotenoid retention typically ≥90%, real-world use may vary). Certifications: ISO 9001, FSSC 22000, Halal, Kosher (typical for this class; verify batch COA). Origin: Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone. Application scenarios Direct compression tablets (high hardness, low friability) Hard-shell capsules and stick packs Cold-water beverage sachets and nutrition blends Vegetarian gummies and chewables (gelatin-free matrix helps) Vendor landscape (quick take) Vendor Certs Lead time Customization Stability/Dispersion Finutra (origin: Hebei, CN) ISO, FSSC, Halal, Kosher ≈ 2–4 weeks Ratios, mesh, carriers Robust; T90 ≈ 30 s Generic Trader A ISO (varies) ≈ 4–6 weeks Limited Inconsistent Contract Mfg B FSSC, GMP Project-based Extensive (MOQ ↑) Good, cost ↑ Customization options Adjust lutein:zeaxanthin:lycopene ratios, choose beadlet size (16–40 mesh), select carriers (e.g., modified starch/alginate), and request nitrogen-flushed, light-barrier packaging. Small digression: color targets can be tuned—handy for brand managers chasing a specific Pantone. Two quick case notes Tablet brand in the EU swapped in these beadlets; friability dropped from 1.0% to 0.3% and dissolution stayed clean. “No more orange dust on the line,” the ops lead joked. A beverage sachet maker reported zero clumping in pilot runs and kept the vegetarian claim intact. Standards and references: 1) USP elements by ICP-MS; 2) ICH Q1A(R2) stability; 3) AOAC carotenoid methods; 4) relevant USP/EP monographs for lutein/lycopene. USP General Chapter Elemental Impurities—Procedures. ICH Q1A(R2): Stability Testing of New Drug Substances and Products. AOAC Official Methods for Carotenoids (e.g., 2016.13). USP/FCC Monographs: Lutein, Lycopene; Ph. Eur. relevant monographs.
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








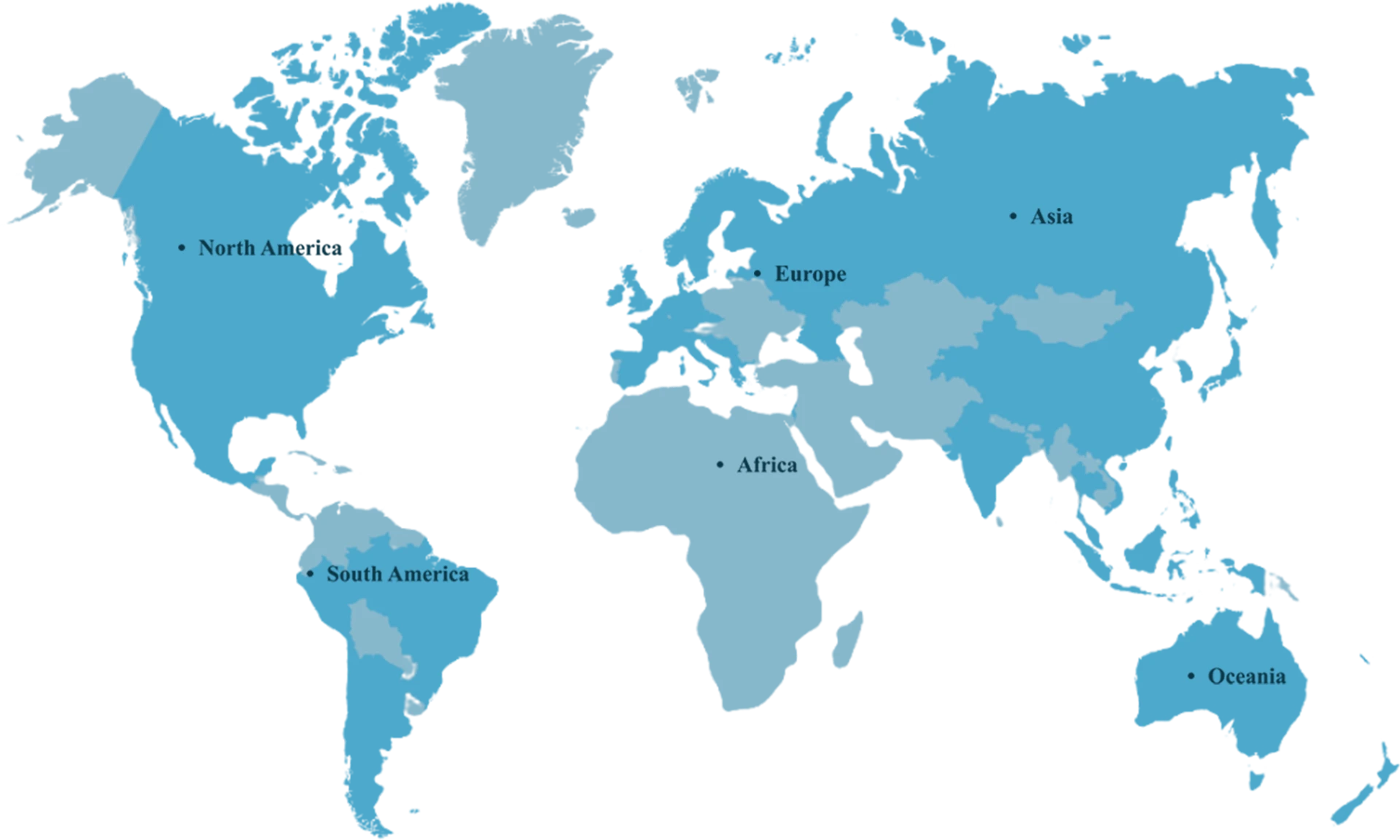
Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy